Dinoprostone : Package Insert / Prescribing Info
Package insert / product label
Dosage form: vaginal insert
Drug class: Uterotonic agents
Medically reviewed by Drugs.com. Last updated on Mar 25, 2024.
On This Page
Dinoprostone Description
Dinoprostone Vaginal Insert is a thin, flat, polymeric slab which is rectangular in shape with rounded corners contained within the pouch of an off-white knitted polyester retrieval system. Each slab is buff colored, semitransparent and contains 10 mg of dinoprostone in a hydrogel insert. An integral part of the knitted polyester retrieval system is a long tape designed to aid retrieval at the end of the dosing interval or earlier if clinically indicated. The finished product is a controlled release formulation which has been found to release dinoprostone in vivo at a rate of approximately 0.3 mg/hr.
The chemical name for dinoprostone (commonly known as prostaglandin E2 or PGE2) is 11α,15S-dihydroxy-9-oxo-prosta-5Z,13E-dien-1-oic acid and the structural formula is represented below:
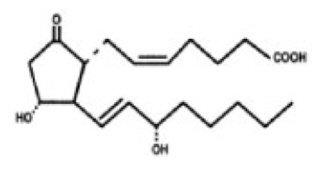
The molecular formula is C20H32O5 and its molecular weight is 352.5. Dinoprostone occurs as a white to off-white crystalline powder. It has a melting point within the range of 65° to 69°C. Dinoprostone is soluble in ethanol and in 25% ethanol in water. Each insert contains 10 mg of dinoprostone in 241 mg of a cross-linked polyethylene oxide/urethane polymer which is a semiopaque, beige colored, flat rectangular slab measuring 29 mm by 9.5 mm and 0.8 mm in thickness. The insert and its retrieval system, made of polyester yarn, are nontoxic and when placed in a moist environment, absorb water, swell, and release dinoprostone.
Dinoprostone - Clinical Pharmacology
Dinoprostone (PGE2) is a naturally-occurring biomolecule. It is found in low concentrations in most tissues of the body and functions as a local hormone (1-3). As with any local hormone, it is very rapidly metabolized in the tissues of synthesis (the half-life estimated to be 2.5-5 minutes). The rate limiting step for inactivation is regulated by the enzyme 15-hydroxyprostaglandin dehydrogenase (PGDH) (1,4). Any PGE2 that escapes local inactivation is rapidly cleared to the extent of 95% on the first pass through the pulmonary circulation (1,2).
In pregnancy, PGE2 is secreted continuously by the fetal membranes and placenta and plays an important role in the final events leading to the initiation of labor (1,2). It is known that PGE2 stimulates the production of PGF2α which in turn sensitizes the myometrium to endogenous or exogenously administered oxytocin. Although PGE2 is capable of initiating uterine contractions and may interact with oxytocin to increase uterine contractility, the available evidence indicates that, in the concentrations found during the early part of labor, PGE2 plays an important role in cervical ripening without affecting uterine contractions (5-7). This distinction serves as the basis for considering cervical ripening and induction of labor, usually by the use of oxytocin (8-10), as two separate processes.
PGE2 plays an important role in the complex set of biochemical and structural alterations involved in cervical ripening. Cervical ripening involves a marked relaxation of the cervical smooth muscle fibers of the uterine cervix which must be transformed from a rigid structure to a softened, yielding and dilated configuration to allow passage of the fetus through the birth canal (11-13). This process involves activation of the enzyme collagenase, which is responsible for digestion of some of the structural collagen network of the cervix (1,14). This is associated with a concomitant increase in the amount of hydrophilic glycosaminoglycan, hyaluronic acid and a decrease in dermatan sulfate (1). Failure of the cervix to undergo these natural physiologic changes, usually assessed by the method described by Bishop (15,16), prior to the onset of effective uterine contractions, results in an unfavorable outcome for successful vaginal delivery and may result in fetal compromise. It is estimated that in approximately 5% of pregnancies the cervix does not ripen normally (17). In an additional 10-11% of pregnancies, labor must be induced for medical or obstetric reasons prior to the time of cervical ripening (17).
The delivery rate of PGE2 in vivo is about 0.3 mg/hour over a period of 12 hours. The controlled release of PGE2 from the hydrogel insert is an attempt to provide sufficient quantities of PGE2 to the local receptors to satisfy hormonal requirements. In the majority of patients, these local effects are manifested by changes in the consistency, dilatation and effacement of the cervix as measured by the Bishop score. Although some patients experience uterine hyperstimulation as a result of direct PGE2- or PGF2α- mediated sensitization of the myometrium to oxytocin, systemic effects of PGE2 are rarely encountered. The insert is fitted with a biocompatible retrieval system which facilitates removal at the conclusion of therapy or in the event of an adverse reaction.
No correlation could be established between PGE2 release and plasma concentrations of PGEm. The relative contributions of endogenously and exogenously released PGE2 to the plasma levels of the metabolite PGEm could not be determined. Moreover, it is uncertain as to whether the measured concentrations of PGEm reflect the natural progression of PGEm concentrations in blood as birth approaches or to what extent the measured concentrations following PGE2 administration represent an increase over basal levels that might be measured in control patients.
Indications and Usage for Dinoprostone
Dinoprostone Vaginal Insert 10 mg is indicated for the initiation and/or continuation of cervical ripening in patients at or near term in whom there is a medical or obstetrical indication for the induction of labor.
Contraindications
Dinoprostone Vaginal Insert is contraindicated in:
- Patients with known hypersensitivity to prostaglandins.
- Patients in whom there is clinical suspicion or definite evidence of fetal distress where delivery is not imminent.
- Patients with unexplained vaginal bleeding during this pregnancy.
- Patients in whom there is evidence or strong suspicion of marked cephalopelvic disproportion.
- Patients in whom oxytocic drugs are contraindicated or when prolonged contraction of the uterus may be detrimental to fetal safety or uterine integrity, such as previous cesarean section or major uterine surgery (see PRECAUTIONS and ADVERSE REACTIONS).
- Patients already receiving intravenous oxytocic drugs.
- Multipara with 6 or more previous term pregnancies.
Warnings
For hospital use only
Dinoprostone Vaginal Insert should be administered only by trained obstetrical personnel in a hospital setting with appropriate obstetrical care facilities.
Women aged 30 years or older, those with complications during pregnancy and those with a gestational age over 40 weeks have been shown to have an increased risk of postpartum disseminated intravascular coagulation. In addition, these factors may further increase the risk associated with labor induction (See ADVERSE REACTIONS, Post-marketing surveillance). Therefore, in these women, use of dinoprostone should be undertaken with caution. Measures should be applied to detect as soon as possible an evolving fibrinolysis in the immediate post-partum period.
The Clinician should be alert that use of dinoprostone may result in inadvertent disruption and subsequent embolization of antigenic tissue causing in rare circumstances the development of Anaphylactoid Syndrome of Pregnancy (Amniotic Fluid Embolism).
Precautions
1. General Precautions
Since prostaglandins potentiate the effect of oxytocin, Dinoprostone Vaginal Insert must be removed before oxytocin administration is initiated and the patient's uterine activity carefully monitored for uterine hyperstimulation. If uterine hyperstimulation is encountered or if labor commences, the vaginal insert should be removed. Dinoprostone Vaginal Insert should also be removed prior to amniotomy.
Dinoprostone Vaginal Insert is contraindicated when prolonged contraction of the uterus may be detrimental to fetal safety and uterine integrity. Therefore, Dinoprostone Vaginal Insert should not be administered to patients with a history of previous cesarean section or uterine surgery given the potential risk for uterine rupture and associated obstetrical complications, including the need for hysterectomy and the occurrence of fetal or neonatal death.
Caution should be exercised in the administration of Dinoprostone Vaginal Insert for cervical ripening in patients with ruptured membranes, in cases of non-vertex or non-singleton presentation, and in patients with a history of previous uterine hypertony, glaucoma, or a history of childhood asthma, even though there have been no asthma attacks in adulthood.
Uterine activity, fetal status and the progression of cervical dilatation and effacement should be carefully monitored whenever the Dinoprostone Vaginal Insert is in place. With any evidence of uterine hyperstimulation, sustained uterine contractions, fetal distress, or other fetal or maternal adverse reactions, the vaginal insert should be removed. An increased risk of post-partum disseminated intravascular coagulation has been described in patients whose labor was induced by physiologic means, either with dinoprostone or oxytocin.
2. Drug Interactions
Dinoprostone Vaginal Insert may augment the activity of oxytocic agents and their concomitant use is not recommended. A dosing interval of at least 30 minutes is recommended for the sequential use of oxytocin following the removal of the Dinoprostone Vaginal Insert. No other drug interactions have been identified.
3. Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity and fertility studies have not been conducted with Dinoprostone Vaginal Insert. No evidence of mutagenicity has been observed with prostaglandin E2 in the Unscheduled DNA Synthesis Assay, the Micronucleus Test, or Ames Assay.
4. Pregnancy
Teratogenic Effects
Pregnancy Category C
Prostaglandin E2 has produced an increase in skeletal anomalies in rats and rabbits. No effect would be expected clinically, when used as indicated, since Dinoprostone Vaginal Insert is administered after the period of organogenesis. Prostaglandin E2 has been shown to be embryotoxic in rats and rabbits, and any dose that produces sustained increased uterine tone could put the embryo or fetus at risk.
Adverse Reactions/Side Effects
Dinoprostone Vaginal Insert is well tolerated. In placebo-controlled trials in which 658 women were entered and 320 received active therapy (218 without retrieval system, 102 with retrieval system), the following events were reported.
| Controlled Studies* | ||
| Active | Placebo | |
| Uterine hyperstimulation with fetal distress | 2.8% | 0.3% |
| Uterine hyperstimulation without fetal distress | 4.7% | 0% |
| Fetal Distress without uterine hyperstimulation | 3.8% | 1.2% |
| N | 320 | 338 |
| STUDY 101-801† | ||
| Active | Placebo | |
| Uterine hyperstimulation with fetal distress | 2.9% | 0% |
| Uterine hyperstimulation without fetal distress | 2.0% | 0% |
| Fetal Distress without uterine hyperstimulation | 2.9% | 1.0% |
| N | 102 | 104 |
Drug related fever, nausea, vomiting, diarrhea, and abdominal pain were noted in less than 1% of patients who received Dinoprostone Vaginal Insert.
In study 101-801 (with the retrieval system) cases of hyperstimulation reversed within 2 to 13 minutes of removal of the product. Tocolytics were required in one of the five cases.
In cases of fetal distress, when product removal was thought advisable there was a return to normal rhythm and no neonatal sequelae.
Five minute Apgar scores were 7 or above in 98.2% (646/658) of studied neonates whose mothers received Dinoprostone Vaginal Insert. In a report of a 3 year pediatric follow-up study in 121 infants, 51 of whose mothers received Dinoprostone Vaginal Insert, there were no deleterious effects on physical examination or psychomotor evaluation (18).
Post-marketing surveillance
Immune System Disorders: Hypersensitivity
Blood and lymphatic system disorders: Disseminated Intravascular Coagulation (See Warnings Section)
Reproductive system: Reports of uterine rupture have been reported in association with use of Dinoprostone Vaginal Insert some required a hysterectomy and some resulted in subsequent fetal or neonatal death.
Vascular Disorders: Hypotension
Pregnancy, Puerperium and Perinatal Conditions: Amniotic fluid embolism
Drug Abuse and Dependence
No drug abuse or dependence has been seen with the use of Dinoprostone Vaginal Insert.
Overdosage
Dinoprostone Vaginal Insert is used as a single dosage in a single application. Overdosage is usually manifested by uterine hyperstimulation which may be accompanied by fetal distress, and is usually responsive to removal of the insert. Other treatment must be symptomatic since, to date, clinical experience with prostaglandin antagonists is insufficient.
The use of beta-adrenergic agents should be considered in the event of undesirable increased uterine activity.
Dinoprostone Dosage and Administration
The dosage of dinoprostone in the vaginal insert is 10 mg designed to be released at approximately 0.3 mg/hour over a 12 hour period. Dinoprostone Vaginal Insert should be removed upon onset of active labor or 12 hours after insertion.
Dinoprostone Vaginal Insert is supplied in an individually wrapped aluminium/polyethylene package with a "tear mark" on one side of the package. The package should only be opened by tearing the aluminium package along the tear mark. The package should never be opened with scissors or other sharp objects which may compromise or cut the knitted polyester pouch that serves as the retrieval system for the polymeric slab.
Dinoprostone Vaginal Insert must be kept frozen until use, and is administered by placing one unit transversely in the posterior fornix of the vagina immediately after removal from its foil package. The insertion of the vaginal insert does not require sterile conditions. The vaginal insert must not be used without its retrieval system. There is no need for previous warming of the product. A minimal amount of water-miscible lubricant may be used to assist insertion of Dinoprostone Vaginal Insert. Care should be taken not to permit excess contact or coating with the lubricant which could prevent optimal swelling and release of dinoprostone from the vaginal insert. Patients should remain in the recumbent position for 2 hours following insertion, but thereafter may be ambulatory. If the patient is ambulatory, care should be taken to ensure the vaginal insert remains in place. If uterine hyperstimulation is encountered or if labor commences, the vaginal insert should be removed. Dinoprostone Vaginal Insert should also be removed prior to amniotomy.
Upon removal of Dinoprostone Vaginal Insert, it is essential to ensure that the slab has been removed, as it will continue delivering the active ingredient. This is accomplished by visualizing the knitted polyester retrieval system and confirming that it contains the slab. In the rare instance that the slab is not contained within the polyester retrieval system, a vaginal exam should be performed to remove the slab.
How is Dinoprostone supplied
Dinoprostone Vaginal Insert (NDC 55566-9500-1) contains 10 mg dinoprostone. The product is wound and enclosed in an aluminium/polyethylene pack.
Store in a freezer: between -20°C and -10°C (-4°F and 14°F). Dinoprostone Vaginal Insert is packed in foil and is stable when stored in a freezer for a period of three years. Vaginal inserts exposed to high humidity will absorb moisture from the air and thereby alter the release characteristics of dinoprostone. Once used, the vaginal insert should be discarded.
Clinical Studies
| Primip/Nullip | Multip | |||||
|---|---|---|---|---|---|---|
| Parameter | Study # | Dinoprostone Vaginal Insert | Placebo | Dinoprostone Vaginal Insert | Placebo | P-Value |
|
||||||
| Treatment Success* | 101-103 (N=81) | 65% | 28% | 87% | 29% | <0.001 |
| 101-003 (N=371) | 68% | 24% | 77% | 24% | <0.001 | |
| 101-801 (N=206) | 72% | 48% | 55% | 41% | 0.003 | |
| Time to Delivery (hours) | ||||||
| Average Median | 101-103 (N=81) | 33.7 | 48.6 | 14.0 | 28.6 | 0.001 |
| 25.7 | 34.5 | 12.3 | 24.6 | |||
| Average Median | 101-801 | 31.1 | 51.8 | 52.3 | 45.9 | <0.001 |
| (N=206 | 25.5 | 37.2 | 20.8 | 27.4 | ||
| Time to Onset of Labor (hrs) | ||||||
| Average Median | 101-103 (N=81) | 19.9 | 39.4 | 6.8 | 22.4 | <0.001 |
| 12.0 | 19.2 | 6.9 | 18.3 | |||
References
- Physiology of Labor In: Williams Obstetrics. Eds. Pritchard, J.A., MacDonald, P.C., and Gant, N.F. Appleton-Century-Crofts, Conn, Pp 295-321, (1985).
- Rall, T.W. and Schliefer, L.S. Oxytocin, prostaglandin, ergot alkaloids, and other drugs; tocolytics agents, In: The Pharmacological Basis of Therapeutics. Eds. Gilman, A.G., Goodman, L.S., Rall, T.W., and Murad, F. MacMillan, Publ. Co., New York, Pp. 926-945, (1985).
- Casey, M.L. and MacDonald, P.C. The initiation of labor in women: Regulation of phospholipid and arachidonic acid metabolism and of prostaglandin production. Semin. Perinat. 10: 270-275, (1986).
- Casey, M.L., MacDonald, P.C. and Mitchell, M.D. Stimulation of prostaglandin E2 production in amnion cells in culture by a substance(s) in human fetal urine. Biochem. Biophys. Res. Comm. 114:1056, (1983).
- Olson, D.M., Lye, S.J., Skinner, K., and Challis, J.R.G. Prostanoid concentrations in maternal/fetal plasma and amniotic fluid and intrauterine tissue prostanoid output in relation to myometrial contractility during the onset of adrenocorticotropin-induced preterm labor in sheep. 116: 389-397, (1985).
- Ledger, W.L., Ellwood, D.A., and Taylor, M.J. Cervical softening in late pregnant sheep by infusion of prostaglandin E-2 into cervical artery. J. Reprod. Fert. 69, 511-515, (1983).
- Olson, D.M., Lye, S.J., Skinner, K., and Challis, J.R.G. Early changes in prostaglandin concentrations in ovine maternal and fetal plasma, amniotic fluid and from dispersed cells of intrauterine tissues before the onset of ACTH-induced pre-term labor. J. Reprod. Fert. 71: 45-55, (1984).
- Caldeyro-Barcia, R. and Posiero, J. Oxytocin and the contractility of the human uterus, Ann, N.Y. Acad. Sci. 75:813, (1959).
- Posiero, J. and Noriega-Guerra, L. Dose-response relationships in uterine effects of oxytocin infusion. Oxytocin. Eds., Caldeyro-Barcia, R. and Heller, J. Pergamon Press, New York, (1961).
- Cibils, L. Enhancement of induction of labor. In: Risks in the Practice of Modern Obstetrics. Aldjem, S. Ed. Mosby Publishing, St. Louis, (1972).
- Bryman, I., Lindblom, B., and Norstrom, A. Extreme sensitivity of cervical musculature to prostaglandin E2 in early pregnancy. Lancet, 2:1471, (1982).
- Thiery, M. Induction of labor with prostaglandins. In: Human Parturition. Eds. Keirse, M.J.N.C., Anderson, A.B.M., and Gravenhorst, J.B. Martinus Nijhoff Publ., Boston, 155-164, (1979).
- Thiery, M. and Amy, J.J. Induction of labor with prostaglandins. In:Advances in Prostaglandin Research. Prostaglandin and Reproduction. Karim, S.M.M., Ed., MTP, Lancaster, Pp. 149-228, (1975).
- MacLennan, A.H., Katz, M., and Creasey, R. The morphologic characteristics of cervical ripening induced by the hormones relaxin and prostaglandin F2 in a rabbit model. Am. J. Obstet. Gynecol, 152:910696, (1985).
- Bishop, E. Elective induction of labor. Obstet. & Gynecol. 5: 519-527, (1955).
- Bishop, E. Pelvic scoring for elective induction. Obstet. & Gynecol. 24: 266-268, (1969).
- Thiery, M. Preinduction cervical ripening. In: Obstetrics and Gynecology Annual, Vol. 12, Ed. Wynn, R.M. Appleton-Century-Crofts, New York, Pp. 103-146, (1983).
- MacKenzie, I.; Information on File: Ferring Controlled Therapeutics Limited (Scotland).
- De Abajo FJ et al. Labor induction with dinoprostone or oxytocin and postpartum disseminated intravascular coagulation: a hospital-based case-control study. Am J Obs Gynecol, 2004, 191: 1637-1643.
Mfg by:
Ferring Controlled Therapeutics Limited
East Kilbride, Scotland, G74 5PB
Made in the U.K.
Distributed by:
Ferring Pharmaceuticals Inc.
Parsippany, NJ 07054
Rev. 08/2013
6765-01
PRINCIPAL DISPLAY PANEL - Pouch Carton
NDC 55566-9500-1
DINOPROSTONE 10 mg
vaginal insert
Contains: One Vaginal Insert containing
10 mg Dinoprostone in 241 mg hydrogel polymer
(cross-linked polyethylene oxide/urethane)
with polyester retrieval system.
Store in a freezer: between -20°C and -10°C (-4°F and 14°F)

| DINOPROSTONE
dinoprostone insert |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Ferring Pharmaceuticals Inc. (103722955) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ferring Controlled Therapeutics Ltd | 298229634 | MANUFACTURE(55566-9500) | |
More about dinoprostone topical
- Check interactions
- Compare alternatives
- Reviews (18)
- Side effects
- Dosage information
- During pregnancy
- Drug class: uterotonic agents
- Breastfeeding
- En español
Patient resources
Professional resources
Other brands
Cervidil, Prepidil, Prostin E2
